Do we already have the power to eradicate genetic disease?
How CRISPR gene-editing technology is revolutionizing medicine
Researchers at the Northwestern Center for Genetic Medicine use the gene-editing technology CRISPR to isolate and alter disease-causing mutations in DNA
March 6, 2019
Are the first children of a new generation, free from inheritable and genetic disease, already born?
Do we already possess the technology to eradicate muscular dystrophy and cystic fibrosis, edit out sickle cell anemia, and prevent contraction of HIV?
The international scientific community has condemned He Jiankui, a Chinese professor, who claims to have used CRISPR, a gene-editing technology, to engineer the world’s first genetically-edited babies. Jiankui claims to have altered twin girls’ embryonic DNA to eliminate a specific gene, making them resistant to contracting HIV.
Dr. Eugene Wyatt is a research assistant professor at the Northwestern Center for Genetic Medicine. He believes Jiankui’s actions were inexcusable because not enough research has been conducted into potential side effects associated with gene-editing technology, called off-target mutations.
CRISPR uses “molecular scissors” to cut, replace or alter specific strands of DNA. However, the scissors in the past have proven unpredictable, causing off-target mutations in the DNA. These unexpected damages can cause problems later in life and be inherited by future generations.
“The big leap for CRISPR is inevitable,” said Wyatt. “How fast do we want the technology to move? What ethical steps should we take along the way before leaping into something like embryonic editing?”
Wyatt researches for the Transgenic and Targeted Mutagenesis Core, specializing in using CRISPR to make mouse models of human diseases. This enables researchers to better understand each disease and engage in drug or therapy development for future applications.
He described the human genome as a basic alphabet constructed with different based-pairs instructing a cell to make proteins. The four fundamental bases (A, C, T and G) in the DNA are arranged allowing different ways of producing proteins, giving the individual different genetic traits. Each protein has a unique signature providing a specific function, shape and localization.
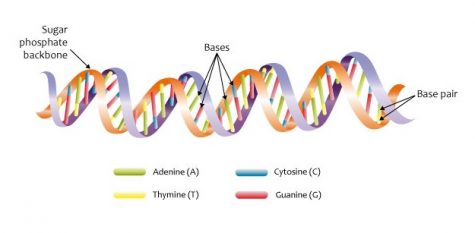
“If a person has 900 specific single-based pairs encoding gene A, a deletion, or mutation in one of those specific pairs, makes the gene non-functional or dysfunctional,” said Wyatt. “CRISPR focuses on the gene’s signature and allows edits of specific regions of the genome with immense precision.”
In 2015, a similar gene-editing technology, TALENs was used in London to reverse an aggressive form of leukemia. The technology ZFNs was used in California to edit out a gene called CCR5, which HIV uses to infect cells. CCR5 is the same gene Jiankui used CRISPR to eliminate in the embryos of the twin girls.
Wyatt said CRISPR avoids the targeting restrictions the other technologies face and makes precision edits to almost any region on the genome.
Wyatt’s core directly injects mouse embryos using CRISPR, or tests on mouse embryonic stem cells to evaluate the consequences of gene-editing actions. Their technology is so precise, researchers can place a fluorescent tag on the end of a specific gene to study where, and when, the gene is expressed in the cell.
“People come to our core with genes that cause cancer, cystic fibrosis and different types of muscular dystrophy,” said Wyatt. “Our research enables us to make disease-specific cell-lines and correct the disease mutation. We then observe how this CRISPR-edited cell-line functions. Is it truly repaired? What are the potential downstream effects?”
The public’s hesitation to embrace the technology centers on the unknown risks.
“The research we’re doing on mice is what scares people for human application,” said Wyatt. “We’re creating germ-line edits that will be propagated to the next generation.”
The public also fear CRISPR engineering will lead to the practice of eugenics – controlled breeding to design a superior human race. The ethical concern is “designer babies” will create a world of genetic discrimination with vast inequality amongst who can, and cannot, afford to create children with desirable physical traits.
Thomas Kulanjiyil, professor of philosophy and teacher of bioethics at the College of DuPage, believes the ethical community must continue to exercise caution when applying the technology to human reproduction.
“If one person in China without the approval of his university can do this, who has the ability to do it next?” asked Kulanjiyil.
“As we develop gene-editing, who will control the ethical and applicable parameters of such a technology? When we are ready to use the technology, are we considering all the safeguards and scrutinizing all the potential effects?”

Kulanjiyil believes if the ethics and medical communities work in tandem, gene-editing can represent a dynamic future with the potential prevention of certain inheritable diseases.
“Society will never totally agree on applying scientific advancement, but if we can responsibly mitigate risks, progression is inevitable,” said Kulanjiyil.
Wyatt said gene-editing technology is moving at such an unprecedented pace, it’s crucial to bring the public into the discussion.
“Are designer babies with genetically selected hair and eye colors two-years away?” asked Wyatt. “Absolutely not. However, applicable uses ex vivo to treat diseases like Duchenne muscular dystrophy could be two years away.”
Ex vivo involves extracting cells out of the patient and editing the cells through CRISPR. The cells are then thoroughly analyzed for potential off-target effects and inserted back into the patient carrying the corrected genes. Wyatt explained ex vivo carries less risk of potential off-target mutations than altering embryonic stem cells.
“The public have the ethical responsibility to determine what level of risk for each drug or therapy we deem acceptable,” said Wyatt. “With control and slow-based implementation, we can appreciate CRISPR’s full possibilities and what unlocking the human genome really means.”
Kulanjiyil said some objectors see the process of manipulating the human genome as not simply a modification, but an unnatural overreach. They fear an alteration will transform what it means to be human.
“We have to constantly ask ourselves, ‘What are we trying to lead the species to?” said Kulanjiyil. “Are we merely trying to eradicate damaging diseases, or are we trying to create a better gene-pool and eliminate undesirable human traits?”
Kulanjiyil said with our rapidly changing world, ethics must provide a consistent bedrock for our human reasoning.
“Ethics provide humans a moral basis, and as science and technology evolve, ethics must always decide what is best for the human community,” said Kulanjiyil.
CRISPR technology is being adapted for use in fields as diverse as agricultural development to medical research and therapeutic application. Wyatt’s lab relies on the precision of CRISPR to research muscular dystrophy cases with a specific-point mutation, requiring a singular base-pair alteration in the DNA. The ease of CRISPR’s application allows researchers to edit multiple genes simultaneously if required by the disease.
Wyatt said CRISPR was discovered when researchers noticed little repeat sequences at the end of bacterial genomes. The scientists discovered the sequences represented a bacterial immunity against invading phages.
He said bacteria can get sick by phages just like humans. CRISPR represents immunity through a type of inoculation. The bacteria evolved to chop up an invading phage and incorporate little pieces of the phage’s DNA into its own genome. When threatened of infection by a similar phage, the bacteria would send out CRISPR machinery of its own DNA, to infiltrate the intruding phage’s DNA and chop it up to prevent infection.
“When we link the endonuclease enzyme Cas9 with a specific DNA sequence, it’s like scissors that can cut wherever we intend,” said Wyatt. “The beauty is in the simplicity. The possibilities are endless.”
Wyatt is excited by the potential therapeutic applications of CRISPR as a gene-therapy. He said unlike current therapies that only treat the symptoms but not the disease itself, CRISPR theoretically permits permanent corrections in the patient’s edited genes.
Wyatt said further research is required to tell if introducing a corrected gene in a patient with a disease, like muscular dystrophy, will correct all the other negative effects associated with years of living with the illness.
“CRISPR will allow us to create some cell-based therapies that are amazingly powerful,” said Wyatt. “From public institutions to private enterprises, there’s currently a major boom in the biotech industry resembling the dynamics of the space-race.”
Wyatt believes every day new physicians and researchers are coming in with a greater appreciation and ability to manipulate the human genome. As a society, we are opening more possibilities and manifesting more power than ever before. We are on the cusp of revolutionizing mankind’s relationship with its own destiny.
“This evolution is happening, whether we are ready or not,” said Wyatt. We must continue to exercise caution and work together. The CRISPR train is coming down the track.”